Prevention
Modern medicine is increasingly transitioning towards preventive care. This shift towards prevention has also been observed in breast cancer care in recent years, particularly with the discovery of the BRCA gene. Subsequently, multiple genes and risk factors have been identified. Depending on these factors, a personalized screening strategy can be chosen. Therefore, it is crucial to understand these genetic and risk factors.
Diagnosis
I was diagnosed with cancer ... This website serves as a portal designed to assist you and your loved ones in accessing personal information and finding solutions to your concerns.
The primary goal of this website is to offer guidance and support to patients as they navigate their journey toward recovery and improved quality of life. The "Diagnosis" section of our website is divided into two main categories. Firstly, under "Anatomy and Physiology," we provide fundamental knowledge about the breast. Secondly, in the "Tumors and Disorders" section, we delve deeper into various breast-related conditions.
Moreover, we aim to provide information to women who may be concerned about potential breast issues but are hesitant to seek immediate medical advice. Knowledge and information can often offer immediate reassurance if a woman is able to identify the issue herself and determine that no specific treatment is necessary. Conversely, we also strive to educate women who have received a diagnosis of a serious breast condition, such as breast cancer, and wish to approach their doctor well-informed and prepared.
Treatment
The treatment for breast cancer should immediately include a discussion about reconstruction. Our foundation has no greater goal than to raise awareness of this among patients and oncological surgeons. By making an informed decision beforehand, we avoid closing off options for later reconstruction while still considering the oncological aspect. Of course, survival is paramount, and the decision of the oncologic surgeon will always take precedence.
The "Reconstruction or not?" page contains all the information you can expect during an initial consultation before undergoing tumor removal. This page is comprehensive, and your plastic surgeon will only provide information relevant to your situation.
"Removing the tumor" details the surgical procedure itself. This is the most crucial operation because effective tumor removal remains paramount. We guide you through the various methods of removal, a decision often made by a multidisciplinary team comprising oncologists, radiologists, pathologists, radiotherapists, breast nurses, gynecologists, oncological surgeons, and plastic surgeons.
The "Breast Reconstruction" section includes information and illustrations of the different reconstruction options along with corresponding steps.
Revalidation
Those treated for cancer often need a long period to recover.
Cancer is a radical illness with a heavy treatment. Often, people have to deal with psychosocial and/or physical problems afterwards, such as stress, anxiety, extreme fatigue, painful joints, reduced fitness, lymphedema... This can have a major impact on general well-being.
There are rehabilitation programmes offered by most hospitals. We cover some of the major topics here.
Quality of life
Quality of life is a key factor in coping with breast cancer. Therefore, it is important to find coping mechanisms that work, which will be different from patient to patient. For some, it may be finding enjoyment in activities they engaged in prior to diagnosis, taking time for appreciating life and expressing gratitude, volunteering, physical exercise... Of prime importance, studies have shown that accepting the disease as a part of one’s life is a key to effective coping, as well as focusing on mental strength to allow the patient to move on with life. In this section we are addressing some topics that patients experience during and after treatment and we are providing information to address them.
Genetic and hereditary breast cancer
Family history as a risk factor for breast cancer
A positive family history is the strongest predictor of a woman developing breast cancer. The more family members diagnosed, the closer their relationship and the earlier the age of onset, the higher the chance of other women in the family also developing the disease.
It is estimated that approximately 20 to 30% of all breast cancer patients have at least one 1st degree relative with the same condition and 5 to 10% of patients have a strong genetic predisposition (more than one relative with breast cancer).
The following factors increase the risk of a woman developing breast cancer:
number of family relatives affected by breast or ovarian cancer,
ratio of affected to non-affected individuals,
how closely related affected relatives are,
the age of onset of the disease,
whether the breast cancer affects one or both breasts (bilateral disease),
the incidence of male breast cancer in the family.
The number of these elements determines whether the risk is high, moderate or low. We also use this data to identify families in whom it may be useful to perform genetic analysis.
The criteria used for genetic analysis are shown in Table 1. These only tend to act as guidelines because occasionally the analysis is also performed in families which do not strictly meet the criteria. For example, if extenuating circumstances exist, such as a family tree that is difficult to assess (a small family or one that includes a large number of male relatives) or where there are significant clinical implications of a positive result. An exception may also be made in the case of extremely anxious patients for whom a genetic test is the only source of reassurance.
Table 1: Inclusion criteria for molecular genetic analysis of the BRCA1 and BRCA2 genes:
families with three first degree relatives* affected by breast and / or ovarian cancer.
families with at least two first and / or second degree relatives ** with breast and / or ovarian cancer occurring in patients who are younger than 50 years of age.
all patients who develop breast and / or ovarian cancer before the age of 35.
relatives belonging to a family in which the mutation has been identified.
male patients with breast cancer.
* First-degree relatives: mother, daughter or sister
** Second-degree relatives: grandmother, grandchild, aunt or niece
Familial and hereditary types of breast cancer
Women with a significant risk of breast cancer frequently have multiple first degree relatives affected by the disease at an early age or have one or more relatives with multifocal or bilateral breast cancer. These women also often have a family history of ovarian cancer and male relatives affected by breast cancer. In these families, the genetic predisposition is the result of inheriting a specific genetic abnormality, involving a mutation of the BRCA1, BRCA2 or BRCA3 gene.Such families usually fulfill the criteria for hereditary breast and ovarian cancer syndrome (see Table).
Tabel: Hereditary breast and ovarian cancer syndrome and familial breast cancer. | |
|
|
| breast cancer in at least two first or second degree relatives at an early age, without fulfilling the criteria for hereditary breast and ovarian cancer syndrome. |
In families where the risk of breast cancer is moderately or just slightly increased, the relationship between affected family members is more distant and the age of diagnosis later. These are examples of familial breast cancers (Table).
In a minority of these families, genetic abnormalities in the BRCA1, BRCA2 or BRCA3 genes are identified but other genetic ‘variations’ may also play a role. These variations have a weaker influence on the risk of developing breast cancer but are probably occur more frequently than the BRCA1, 2 or 3 mutations and may have a greater impact on the general population. There is also the real possibility that the effect of these variants is influenced by non-genetic factors.
Unfortunately, it is not currently possible to perform a molecular analysis for these genome mutations. The risk for unaffected family members has to be calculated based on information obtained by analyzing the family tree.
It may in fact be difficult to distinguish between hereditary and familial forms of breast cancer. The contribution of genetic mutations is best regarded as a continuum: in familial breast cancer genetic abnormalities play a minor role in the risk of breast cancer, whereas in hereditary forms, BRCA1, BRCA2 and BRCA3 mutations have a significant impact. Many families, however, find themselves caught at the boundary between these two entities.
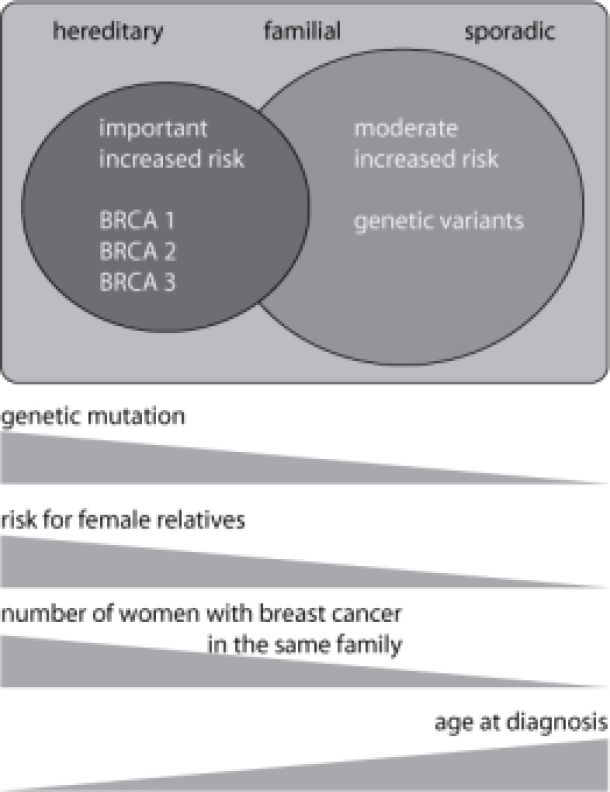
Figure: A Family history of breast cancer helps divide patients into three overlapping categories: sporadic, familial and hereditary. An early age of diagnosis and a large number of affected family members increases the likelihood of the hereditary form. Genetic abnormalities (mutations in the BRCA1, BRCA2 and BRCA3 genes) are most frequently demonstrated in the hereditary form. These genetic defects cause an increased risk of breast and ovarian cancer in female carriers. In familial breast cancer, genetic mutations have less influence on risk.
BRCA1, BRCA2, Breast and ovarian cancer
To understand the genetics of breast cancer, we first need to explain some basic principles. Our genetic information is contained in individual packages called chromosomes. We each have 46 chromosomes which are divided into 23 pairs: 22 pairs of autosomal chromosomes and one pair of sex chromosomes. We inherit one chromosome of each pair from our father (23) and one chromosome of each pair from our mother (23). A chromosome consists of a single long strand of DNA containing our genetic code. A gene is a fragment of DNA, somewhere on the chromosome, that contains the code for a protein with a specific role. The information on each chromosome of the pair is the same, so we have two copies of each gene. BRCA1 and BRCA2 are genes that code for a protein that normally protects the individual against developing cancer. Where there is an error or mutation in one or another of the genes, the risk of developing cancer is greatly increased. Abnormalities in BRCA1 and BRCA2 genes may also be inherited and the chance that an affected individual will pass on the abnormality to his / her child is 50%.
Female carriers of the BRCA1 or BRCA2 mutation have a significant risk of developing breast and ovarian cancer (Table). The risk of breast cancer is probably equal for the carriers of the BRCA1 or BRCA2 mutations, exceeding over 80%. The risk of developing ovarian cancer is significantly higher in female carriers of the BRCA1 mutation.
The cancer risk is likely elevated in male carriers of the mutations, although to a much lesser extent. Men seem to be at a particular risk of developing breast cancer if they are carrying the BRCA2 mutation. This knowledge and the mode of transmission enable us to identify families that may be potential carriers. It includes families with a breast cancer diagnosis at a young age, a history of breast and ovarian cancer, bilateral breast cancer in one or more relatives, or male breast cancer in the family. One can see that these are also the criteria used to identify families at risk of breast cancer.
Tabel: Risks associated with having a mutation in the BRCA1 or BRCA2 gene.* | ||
Female carrier | ||
BRCA 1 | BRCA 2 | |
Breast cancer | 85% | 86% |
Contralateral breast cancer** | 60% | 55% |
Ovarian cancer | 63% | 27% |
Male carrier | ||
BRCA 1 | BRCA 2 | |
Breast cancer | possibly increased | 7% |
Darmkanker | possibly increased | possibly increased |
Prostate cancer | 33% | 34% |
Melanoma | - | 5% |
* These risks are derived from studies of families with significant BRCA1 and BRCA2 mutations and can therefore be seen as a maximum.
** Breast cancer in the other breast of a woman with breast cancer
Patients often wonder if they may be the source of a particular mutation, but this is rarely the case. In almost all instances the mutation is derived from a parent. This explains why the mutation in a BRCA1 or BRCA2 gene can be detected in a large number of people from the same family suffering from breast or ovarian cancer. It also explains why a mutation is almost never detected in patients without a family history, even if they are diagnosed with cancer at a young age.
Mutations in the BRCA1 and BRCA2 genes can occur anywhere along the length of the gene and there are no ‘hot-spots’ or frequently recurring mutations. As a result of the large size of both genes, the molecular analysis is time consuming and intensive: it takes about 3 to 6 months per patient. The complexity of both genes also has a negative influence on the sensitivity of the analysis. The sensitivity depends on the types of mutations that occur in a given population and all forms of the mutation may not be detected using the same technique.
Although both genes can be completely analyzed, one needs to bear in mind that the result may be falsely negative: the particular abnormality of the BRCA1 or BRCA2 gene may not be identified by the technique used.
Risk-reducing strategies for carriers of BRCA1/2 mutations
Women who have inherited mutations in the BRCA1 or BRCA2 genes have substantially increased risks of breast and ovarian cancer. Mutation carriers have various options, including extensive and regular surveillance, risk-reducing surgery and chemoprevention.
The first option is surveillance (breast self-examination, clinical breast examination, screening using mammography and breast magnetic resonance imaging (MRI), trans-vaginal ultrasound scanning and blood levels of CA125). This concept is based on early detection of cancer rather than cancer prevention. Undoubtedly, surveillance is the least invasive option; however, it is associated with various negative consequences, such as increased anxiety, false reassurance and unnecessary biopsies.
Risk-reducing surgery in BRCA1/2 gene mutation carriers includes prophylactic bilateral salpingo-oophorectomy (BSO, removal of the ovaries and fallopian tubes) and/or prophylactic bilateral mastectomy. The aim of surgery is to reduce the risk of cancer development and to reduce mortality. In a large, retrospective analysis of BRCA carriers, BSO was found to have reduced the risk of ovarian cancer by 96% and breast cancer by 53% at a mean follow-up of 9 years. The potential benefit of surgical prophylaxis however has to be weighed against the risk of surgical complications and the impact of mastectomy or oophorectomy on a woman’s self-image and their sexual and reproductive function.
The final option is chemoprevention. It is well recognised that oestrogen plays an essential role in breast cancer development by exerting a carcinogenic effect. Therefore, targeting oestrogen synthesis with anti-oestrogen medication aims to reduce this risk. Currently there are few studies investigating the use of chemoprevention in BRCA1 or BRCA2 mutation carriers and this option remains relatively controversial. Only in women who have already developed breast cancer has Tamoxifen been shown to reduce the risk of developing a new tumour by approximately 50%. Chemoprevention may however have a role for asymptomatic mutation carriers in the future.
Choosing between these different risk-reducing strategies is a complex process involving both personal choice and medical opinion. Important personal factors include the anxiety associated with developing a cancer, the desire to have a family, one’s sexuality and its effect on relationships and any previous experience with cancer in the family. There is also no medical consensus on the benefits of risk-reducing strategies.
The optimal frequency of screening for both breast and for ovarian cancer remains uncertain. Although we know that Magnetic Resonance (MRI) can detect breast cancer at early stage, the role of MRI screening in BRCA1 and BRCA2 gene carriers is controversial. There is currently insufficient data on the improvement in life expectancy offered by the different risk-reducing strategies. For example, although surgery can reduce the chance of developing breast cancer, it is unknown whether this, in comparison to intensive screening, actually results in a better overall survival. Also, the effect of temporary hormonal substitution after prophylactic removal of the ovaries is unclear, because there are fears that the risk of developing breast cancer would rise again.
Therefore when considering the different options, there is no single “best” choice. It is vital that each patient finds a solution that she is most comfortable with. For some, this is risk-reducing surgery, whilst for others regular clinical examination offers the most reassurance. In addition, meeting other affected patients can be helpful.
For male carriers of the BRCA1 or BRCA2 mutation, screening is recommended for the early detection of colon and prostate cancer and skin tumor (table).
Table: possible preventive strategies in carriers of a BRCA1 or BRCA2 mutation | ||
Female carriers | ||
Breast cancer | Screening | monthly self-examination from an early age |
clinical examination by a doctor every 3-6 months from 20 years of age | ||
annual ultrasound and mammography, +/- MRI from 25 years of age | ||
Risk-reducing surgery | prophylactic mastectomy | |
prophylactic bilateral salpingo-oophorectomy | ||
Chemoprevention | amoxifen (currently used in women who have already developped breast cancer) | |
Ovarian cancer | Screening | gynaecological exmaination every 6 months from 35 years of age |
transvaginal ultrasound every 6 months from 35 years of age | ||
blood level of CA-125 every 6 months from 35 years of age | ||
Risk-reducing surgery | prophylactic bilateral salpingo-oophorectomy | |
Chemoprofylaxie | (possible effect of hormonal contraceptives) | |
Male carriers | ||
Breast cancer | Screening | no uniform guidelines |
Bowel cancer | Screening | colonoscopy at 50 years of age |
Prostate cancer | Screening | prostate examination and ultrasound |
blood level of PSA from 40 years of age | ||
Melanoma | Screening | annual skin examination (usually only for BRCA2 gene carriers) |
The CHEK-2 gene
Besides the BRCA-genes, mutations occur in other genes that protect the individual from cancer development. Usually, these mutations have lower penetrance, which means that there is a lesser chance of developing cancer from these mutations. One of the most studied mutations besides BRCA-1 and BRCA-2 is the CHEK2-gene variant. CHEK2 is a gene located on chromosome 22, that encodes for a protein helping us to repair damaged DNA. A mutation in the CHEK2-gene inherited from one of the parents increases the breast cancer risk 2-fold. However, if the mutation exists in both the paternal and maternal inherited genes, the risk increases 6-fold. The gene mutation seems to be more frequent in the northern European populations, with 3% of people carrying the mutation (Finish, Dutch), than in southern European populations (0.5%). Although more rare than the BRCA-mutations, and incorporating a lesser risk for breast cancer, CHEK2 is currently considered the third most important breast cancer susceptibility gene.
References
Apostolou P1, Fostira F. Hereditary breast cancer: the era of new susceptibility genes.. Biomed Res Int. 2013;747318. doi: 10.1155/2013/
D. Easton. CHEK2 ∗1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies,” The American Journal of Human Genetics, 2004;74:1175–1182.
Liu C1, Wang Y, Wang QS, Wang YJ. The CHEK2 I157T variant and breast cancer susceptibility: a systematic review and meta-analysis.. Asian Pac J Cancer Prev. 2012;13:1355-60
S. Narod et al. Estimating survival rates after ovarian cancer among women tested for BRCA1 and BRCA2 mutations. Clinical Genetics 2012; 83:3:232–237
M. Gage, D. Wattendorf, and L. R. Henry. Translational advances regarding hereditary breast cancer syndromes. Journal of Surgical Oncology. 2012;105:5. 444–451
C. Cybulski, B. Gorski, T. Huzarski et al. Effect of CHEK2 missense variant 1157T on the risk of breast cancer in carriers of other CHEK2 or BRCA1 mutations,” Journal ofMedical Genetics 2009; 46:132–135.
The genetic consultation
The evaluation of the usefulness of a genetic counselling test is primarily based on an analysis of the patient's family history. As mentioned, the criteria listed in Table 1 are used for orientation purposes. On the basis of the family history, not only an indication for starting a molecular examination is made, but also an estimation of the risk of breast cancer for other women in the family is made. The latter is particularly important if the hereditary examination does not show any mutation.
If heredity is suspected in a family, heredity testing or also mutation analysis can be initiated. Mutation analysis is initially offered to a person who has already developed breast or ovarian cancer. Molecular genetic testing is offered to as many affected women in a family as possible. The reason for this is as follows: in families in which a BRCA1 or BRCA2 mutation is present, it is still possible for a woman who is not a carrier to develop breast or ovarian cancer. This person has therefore developed cancer without being genetically affected; the scientific name for this phenomenon is phenocopy.
If the test were to be carried out on this woman alone, the family would obviously make the wrong decision. In order to minimise this risk, all affected relatives should be fully examined. The initiation of a hereditary examination in a person who has already developed cancer is called a diagnostic genetic examination. In this genetic testing, we look for the genetic abnormality in the BRCA1 or BRCA2 gene. This search takes about six months, despite recent technological developments. The examination is reimbursed in Belgium.
If a diagnostic test can reveal a mutation, we can check all unaffected relatives who wish to do so to see whether they have inherited the mutation. We call this test a predictive or presymptomatic test. On the basis of this presymptomatic testing, we can be certain about the risk of developing breast or ovarian cancer: women who have not inherited the mutation have a population risk of breast and ovarian cancer, while in women who are carriers, this risk is significantly increased. Of course, it is also useful to offer male relatives this test, since their risk of colon and prostate cancer may be increased and because they can also pass on the mutation to their children. This is obviously very important if they have daughters themselves.
If the diagnostic test in an affected woman cannot detect a mutation, it is not possible to say whether or not breast cancer runs in her family. A false normal test result is one possible explanation. There is also a chance that a mutation in another unknown (and therefore unexamined) gene is present in her family. For genetic advice to her unaffected relatives, we must then fall back on the family history; offering a presymptomatic examination to other relatives is not possible in this case.
If all the women in a family who developed breast or ovarian cancer have died, presymptomatic testing of an unaffected first-degree relative may be considered. However, in these women, the a priori probability of being a carrier is 50%, so a negative test result should be considered uninformative. There is a chance that a BRCA1 or BRCA2 mutation was present in affected relatives, but that person did not inherit the mutation. Moreover, the possibility of a false negative result still exists. If a mutation is found, carrier status is confirmed with certainty.
An important step in the initiation of a hereditary test is obtaining the informed consent of the patient. Given the specific nature of a hereditary test, a number of topics are discussed with the patient before the test is started, so that she is fully informed about the course, implications and possible test results of the hereditary test. Specific points of attention are listed in the table.
Table. Points of attention when obtaining informed consent from the patient when initiating a hereditary test.
the right not to be examined
the purpose, reliability and course of genetic testing
the cost of genetic testing
the implications of normal genetic testing or detection of a genetic abnormality
the possibility that no useful information is obtained after the test is completed
the disadvantages of genetic testing
the confidentiality of test results
the possibility of discrimination
the risk of carrier status in children
In addition to providing information about genetic testing, increasing attention is being paid to the psychological counselling of patients and families in whom testing is initiated, both in diagnostic and in presymptomatic settings. The main objective of this counselling is to offer support tailored to the needs of the patient and her surroundings in
dealing with and processing the test result
exchanging information within a family
choosing a particular preventive strategy
concerns about carrier status in children or other relatives
the impact of hereditary testing on the relationship
the desire to have children
Clinical example
Suzy seeks a medical consultation due to her concerns about the prevalence of breast cancer in her family. She is 27 years old and in good health otherwise. A family tree (see figure) reveals that a significant number of Suzy's paternal relatives have had breast cancer at a young age. Her aunt, Linda, was diagnosed at 38 years old, and Suzy's grandmother succumbed to the same tumor at 42 years old. Additionally, both of Suzy's grandmother's older sisters were diagnosed with breast cancer: one at 65 years old, while the other developed bilateral disease, initially at 45 and then again at 51 years old. Among Suzy's father's five cousins, two also experienced early-onset breast cancer.
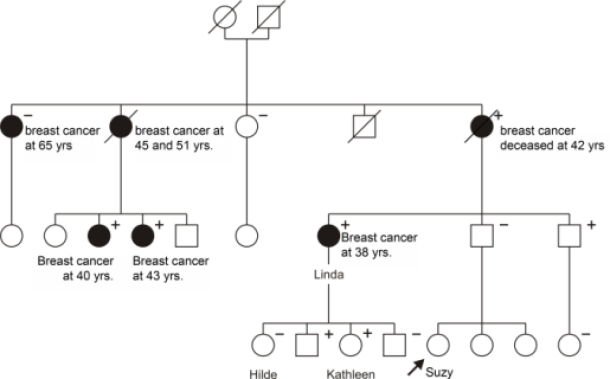
Figure: Hypothetical pedigree depicting a family with a genetic predisposition for breast cancer. Male relatives are represented by squares, while female relatives are represented by circles. Individuals who developed breast or ovarian cancer are shaded black. A line through the symbol indicates the deceased. In this family, many female relatives developed breast cancer at an early age, suggesting a potential hereditary component.
A genetic abnormality in Aunt Linda's BRCA-1 gene was identified. Further examination revealed several other relatives, both male and female, as carriers of this genetic mutation (denoted by a '+'). Some relatives had already been diagnosed with breast cancer (diagnostic test), while others remained unaffected (screening test). In some cases, the genetic mutation was not present (denoted by a '-'). Notably, Suzy's grandmother's eldest sister, diagnosed with breast cancer at 65 years old, tested negative for the mutation, indicating a "phenocopy."
Suzy confirms during the consultation that there is a high likelihood of a hereditary component to her family's breast cancer. Further genetic research is required, but Suzy is surprised that a blood test is not performed immediately. The doctor explains that genetic research typically begins with an individual who has already developed cancer because the chances of finding the genetic mutation are highest. Prior to the consultation, Suzy had already discussed the possibility of genetic testing with Aunt Linda, who responded favorably. Consequently, Suzy decides to make a joint appointment at the genetic clinic to begin testing.
At this appointment, Aunt Linda receives comprehensive information about the details of genetic testing, and her motivation to participate is explored. It transpires that Aunt Linda had already considered a consultation before Suzy's request, explaining her warm response. She had also openly discussed genetic testing with her own children. However, Aunt Linda expresses concern and discomfort about the potential consequences of a positive result, especially given that her children may have inherited the affected gene. Before a blood sample is taken, Aunt Linda signs a consent form verifying that she fully understands the implications of a positive result. Although disappointed that the analysis will take six months, she comes to understand the complexities of the molecular test.
Six months later, Aunt Linda receives a letter confirming the completion of the genetic investigation and scheduling a consultation to discuss the results. Aunt Linda and Suzy agree to attend this appointment together. At the genetic clinic, they are informed that a mutation in the BRCA1 gene has been identified, confirming their suspicions. Although shocked by the news, Aunt Linda had already prepared for this eventuality and decided to have prophylactic removal of both ovaries, but not her breasts. She opts for close oncological follow-up to minimize the risk of breast cancer.
Suzy anticipates an immediate blood test to determine if she is also a carrier. However, the doctor explains that it would be better to first test her father, as he has a 50% chance of being a carrier. If her father does not have the mutation, there is no need to test the rest of his family. Although disappointed, Suzy finds comfort in the possibility that her father may not be a carrier. She is also pleased to hear that her father's investigation will take less time now that the BRCA1 mutation has been identified. Her father is delighted to learn that he is not a carrier, meaning that Suzy and her sisters have not inherited the mutation, and their risk of breast cancer is no higher than that of other women their age.
Aunt Linda's eldest daughter, Hilde, is unsure about undergoing genetic testing and decides to defer. On the other hand, Aunt Linda's youngest daughter, Kathleen, is determined to proceed. She makes an appointment at the genetic clinic, where she receives comprehensive information and meets with a psychologist to discuss the implications of a positive result. Kathleen decides that if she is a carrier, she will undergo prophylactic mastectomies and immediate breast reconstruction. She returns to the clinic four weeks later to receive her results, which confirm that she has inherited the mutation. Despite the initial shock, Kathleen feels empowered by the choices available to her and finds reassurance in the support provided by the clinic.
In the following months, there is discussion within the family about undergoing genetic testing. Some relatives decline, while others, both male and female, express interest. Hilde eventually reaches a decision and learns that she is not a carrier, bringing her relief but also feelings of guilt toward her sister Kathleen.
